Introduction: Invariant natural killer T (iNKT) cells, with their inherent lack of allorecognition, are a promising platform for universal donor cellular therapies. Murine studies have shown that the iNKT cell population is comprised of subsets, similar to T helper cell subsets, with distinct patterns of cytokine expression and functional capacities. The adoptive transfer of iNKT2 or iNKT17 cells suppresses graft-versus-host disease in murine models, while iNKT1 cells have cytotoxic capacity towards malignant cells, suggesting that the composition of iNKT-based cellular therapies could be optimized by using different subsets in different disease settings. However, the heterogeneity of human iNKT cells remains poorly understood, and the phenotypic identity of functionally distinct subsets remains elusive. Herein, we performed single cell RNA-sequencing (scRNA-seq) of purified human iNKT cells from peripheral blood (PB), cord blood (CB), thymus, and bone marrow (BM) to reveal distinct subsets.
Methods: Human iNKT cells were enriched by magnetic bead-based positive selection (Stem Cell Technologies) utilizing anti-Vβ11 antibody (Beckman Coulter), followed by fluorescence activated cell sorting including anti-Vα24 antibody. scRNA-seq libraries were generated using the Rhapsody platform (Becton Dickinson) employing a targeted panel (PB only) or whole transcriptome analysis (all tissues). Data was processed using the BD Rhapsody pipeline (Seven Bridges) followed by analysis in R (Seurat v4.1.0).
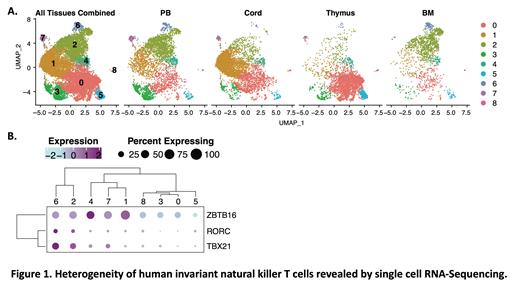
Results: ScRNA-seq of iNKT cells from PB, CB, thymus, and BM revealed 9 transcriptionally distinct clusters derived from cells from each tissue (Fig 1A). While iNKT cells from some tissue sources dominated certain clusters, PB-derived iNKT cells were relatively equally distributed among all clusters. We found that clusters of human iNKT cells expressing TBX21 co-express RORC (Clusters 2 & 6, Fig 1A-B), in contrast to murine iNKT cells in which TBX21 and RORC expression isolates to distinct clusters. iNKT cells in Clusters 2 & 6 also upregulated genes suggesting cytotoxic function as well as receptors important for natural killer cell function, therefore we characterized cells in these two clusters as a Th1/17/NK-like population. Interestingly, the incorporation of oligomer-conjugated antibodies into our analyses allowed the detection that Cluster 6 was CD45RA + with absent CCR7 transcript, similar to that of T effector memory CD45RA + (TEMRA) cells. Other clusters which downregulated both TBX21 and RORC displayed relative upregulation of ZBTB16 and may represent Th2-like iNKT cells (Clusters 1, 4, & 7). iNKT cells from these clusters also expressed IL4R and other genes important for T cell function. Surprisingly, despite excluding CD24 + iNKT precursor cells, we found several clusters with relatively minimal expression of any of these canonical transcription factors (Clusters 0, 3, 5, & 8). These four clusters were also characterized by upregulation of SELL, CCR7, and CD45RA, suggesting a naïve or undifferentiated phenotype, and were especially abundant in the thymus, though found in the periphery as well. We further found that CD4 + iNKT cells were comprised of Th2-like clusters as well as a Th1/17/NK-like cluster (Cluster 2), a population previously described in mice. These results suggest that CD4 expression may not clearly distinguish functionally distinct subsets. Importantly, our results include the first transcriptional analysis of CD8 + human iNKT cells, which (in our PB-only analysis) revealed two distinct CD8 + clusters, one similar to the iNKT1/17/NK-like population and one similar to the naïve/undifferentiated population.
Conclusions: Collectively, these data reveal novel insights into the heterogeneity of human iNKT cells from multiple immunologic tissues. Our transcriptomic data predict that 1) human iNKT cells include a novel TBX21 +/RORC + population not found in mice; 2) naïve/undifferentiated iNKT cells are present not only in the thymus, but also in bone marrow and peripheral blood; and 3) human CD8 + iNKT cells include both Th1/17/NK-like and naïve/undifferentiated populations. These insights provide a critical foundation for optimal use of human iNKT cells in the development of cellular therapies. Studies to correlate transcriptome and function in these novel human iNKT cell populations are underway.
Disclosures
Hollingsworth:insitio: Current Employment. Negrin:Co-Immune: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Regimmune, Inc.: Consultancy; Appia Bio: Membership on an entity's Board of Directors or advisory committees; Biorasi: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Cellenkos: Consultancy; UpToDate: Patents & Royalties; Orca Bio: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Garuda Therapeutics: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal